Cosmetic Definition Tga
Cosmetic Definition Tga, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
The therapeutic goods administration tga regulates medicines and products that are marketed as having a therapeutic effect including most skin whitening lotions primary sunscreens disinfectants complementary medicines and blood products.

Zoya cosmetics. Definition of a cosmetic. The tga only assesses cosmetic products that make therapeutic claims. In this case wed regulate the ingredients as cosmetic ingredients.
This might be because the tga doesnt recognise the therapeutic purpose of your product. If the tga dont regulate your product we might still regulate it. National co ordinating committee on therapeutic goods.
The tga is responsible for conducting assessment and monitoring activities to ensure. For example moisturisers that contain a sunscreening agent as a secondary component and have a stated therapeutic purpose eg. It is important to understand that in australia almost all cosmetic ingredients are regulated as industrial chemicals this includes those described as natural or organic such as oils and extractsthe cosmetic can only be approved for market if.
Tga therapeutic goods administration a division of the federal department of health that is the regulatory body for therapeutic goods including medicines medical devices gene technology and blood products in australia. This document was issued by the national co ordinating committee on therapeutic goods ncctg following input from the cosmetic toiletry and fragrance association of australia to provide guidance in relation to the cosmetictherapeutic interface in respect of product claims. In australia cosmetics that contain sunscreens uv filters are regulated by the health authority therapeutic goods administration tga.
The therapeutic goods administration tga is responsible for administering the therapeutic goods and related manufacturing labelling and advertising requirements. These definitions are current as at 31 october 2018. Advertisers are encouraged check the source of the definition for the current definition as legislation is amended from time to time and may on occasions be replaced or new instruments made.
When non compliant advertising comes to the tgas attention the advertiser is notified. In the first instance the tga seeks to inform educate and assist advertisers to comply with the rules relating to advertising. Helps protect skin from the damaging effects of uv radiation are.
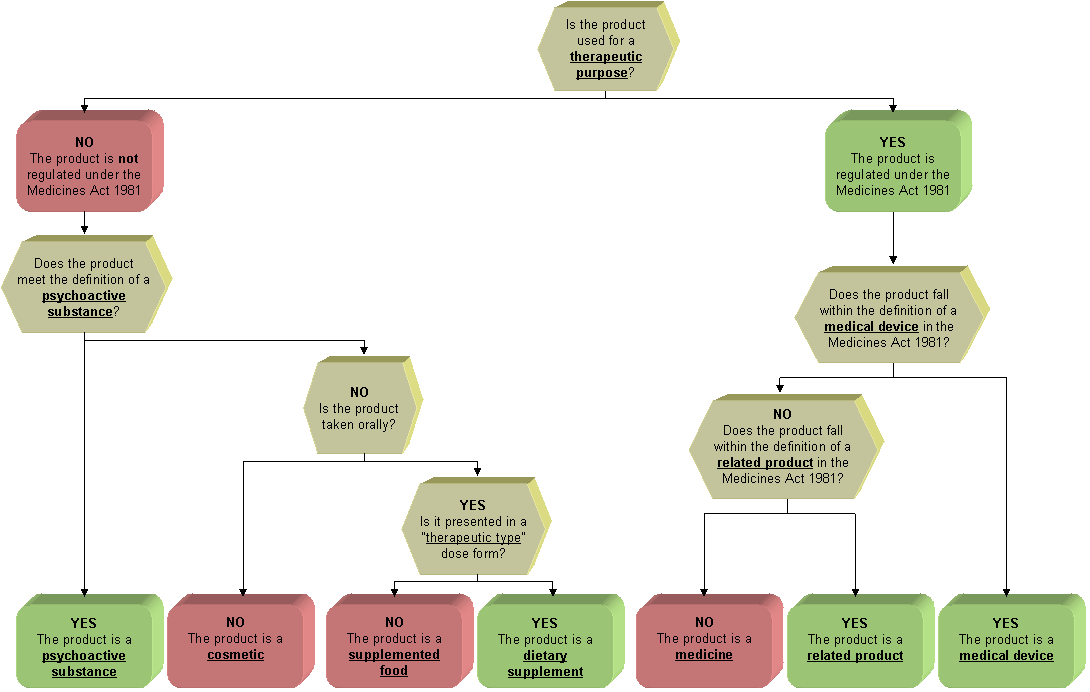
One of the main factors in determining whether a product is a cosmetic or a medicine or a medical device is the claims made about the product. A cosmetic is a substance or preparation that is for use on any external part of the human bodyor inside the mouthto change its appearance cleanse it. You dont need to register with us if any of the following apply.