Cosmetic Definition Us Fda
Cosmetic Definition Us Fda, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
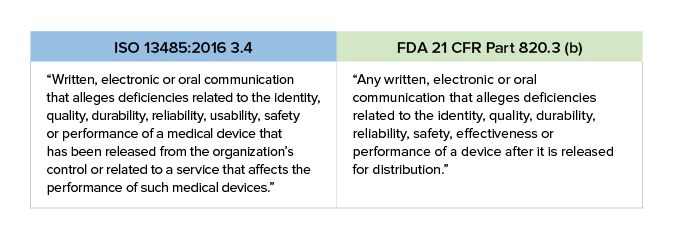
Medical Device Complaint Handling Understanding The Basics Oriel Stat A Matrix Blog Cosmetic Dentistry Hull
Food and drug administration center for food safety and applied nutrition outreach and information center 5001 campus drive hfs 009 college park md 20740 3835.

Cosmetic dentistry hull. The products include skin care personal care cosmetics and fragrance. Senator from new york. Some cosmetics are even classified as drugs making the requirements even stricter and more difficult to comply with.
Whether a product is a cosmetic or a drug under the law is determined by a products intended use. The cosmetics marketed in the united states whether they are manufactured here or are imported from abroad must comply with the labeling requirements of the federal food drug and cosmetic fd. The main competent department for the regulation of cosmetic products is the center for cosmetics regulation and research ccrr which belongs to the philippines fda and has 2 sub branches the licensing and registration division and the product research and standard development division.
Food and drug administration fda to oversee the safety of food drugs medical devices and cosmeticsa principal author of this law was royal s. As labeling is not only used to help inform consumers of a products intended use and any related warnings also. Cosmetics comprise a range of products that are used to care for the face and body or to enhance or change the appearance of the face or body.
Cosmetic labeling what is the key difference between us and eu. And eu require for cosmetic companies who intend to distribute on the market to comply with cosmetic labeling requirements. The vcrp assists fda in carrying out its responsibility to regulate cosmetics.
Part 2 cosmetic products 1 definition. This page provides an overview of cosmetics and the requirements that fda verifiesenforces at the time they are imported or offered for import into the united states. Cosmetic companies may register in the united states through fdas voluntary cosmetic registration program vcrp.
There is a vast array of cosmetics available. The united states federal food drug and cosmetic act abbreviated as ffdca fdca or fdc is a set of laws passed by congress in 1938 giving authority to the us. Cosmereg can assist you in fda cosmetic labeling and ingredient compliance requirements by cross referencing your labeling against the code of federal regulations the federal register.
Each sub category of cosmetics has its own characteristics however the products are generally formulated using a mixture of chemical.








