Cosmetics Regulation Provisions V12
Cosmetics Regulation Provisions V12, Indeed recently has been hunted by consumers around us, perhaps one of you personally. People now are accustomed to using the internet in gadgets to view video and image information for inspiration, and according to the name of this article I will discuss about
If the posting of this site is beneficial to our suport by spreading article posts of this site to social media marketing accounts which you have such as for example Facebook, Instagram and others or can also bookmark this blog page.
The cosmetics regulation specifically bans the use of lead in cosmetics and our industry is in full support of the regulations measures.

Cosmetic brands vietnam. List of amending act and adaptation orders 4. Regulatory provision on sale of cosmetics d. How to obtain license b.
Links to laws and regulations that fda enforces as well as related overviews and reference materials are posted in this. The provision of warnings shall not exempt persons defined in articles 2 and 4 from compliance with the other requirements laid down in this regulation. 3 this regulation aims at simplifying procedures and streamlining terminology thereby reducing administrative burden and ambiguities.
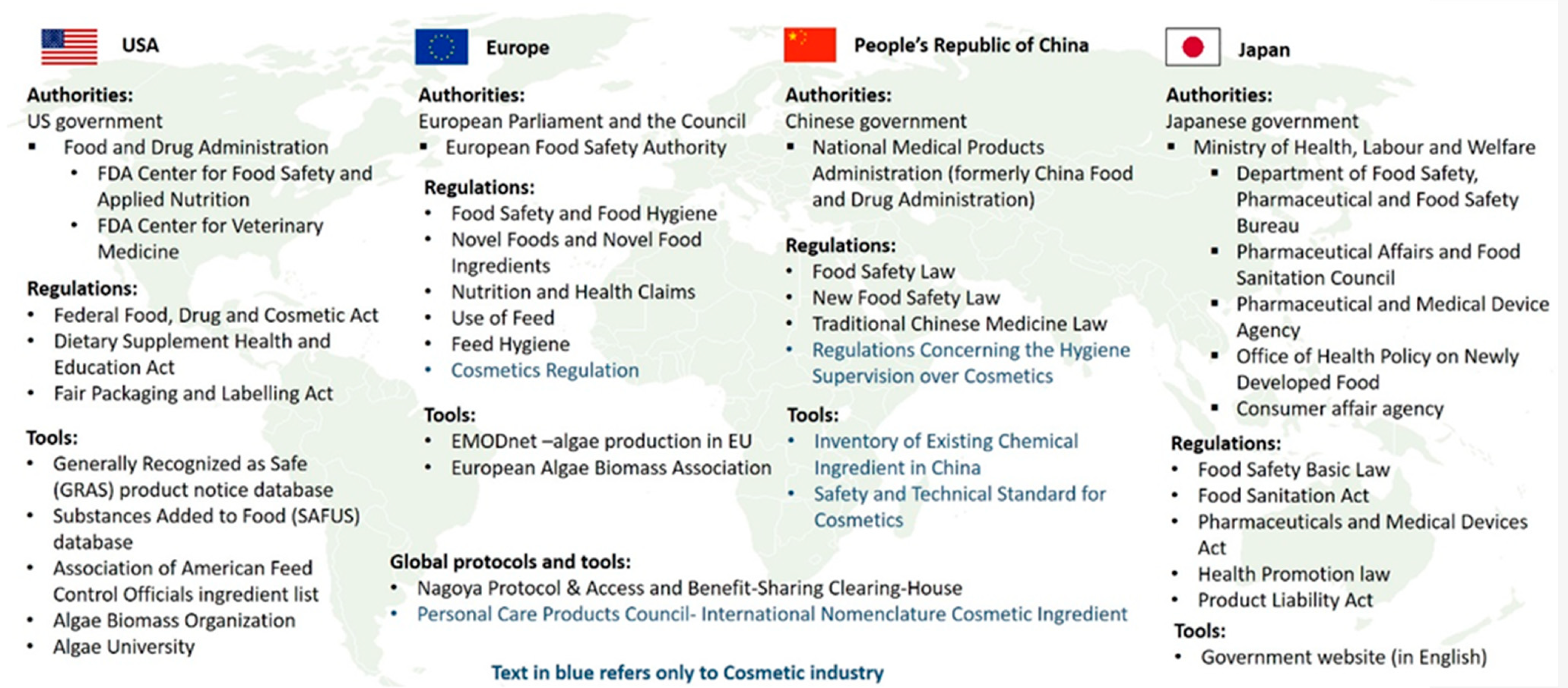
China state council released the final version of cosmetic supervision and administration regulation on june 29 2020 and will implement on january 1 2021the regulation gives clear provisions for filing or registration of cosmetic ingredients and finished products production of cosmetics labeling and advertisement as well as supervision over cosmetics throughout the supply chain. It strengthens the safety of cosmetic products and streamlines the framework for all operators in the sector. Regulation ec n0 12232009 on cosmetic products is the main regulatory framework for finished cosmetic products when placed on the eu market.
Only cosmetic products for which a legal or natural person is designated within the community as responsible person shall be placed on the market. The regulations can create binding obligations and have the force of law. This regulation the maximum concentration of hg remains fixed at 0007 contains thiomersal 16102010 17 phenylmercuric salts including borate phenyl mercuric acetate phenyl mercuric benzoate 62 38 4 94 43 9 200 532 5 202 331 8 eye products 0007 of hg if mixed with other mercurial compounds authorized by this directive the.
College of pharmacy kathora naka amravati maharashtra 444604. Terminology of cosmetics 2. Regulation ensures that legal requirements are imple mented at the same time throughout the community.
Moreover a comprehensive safety assessment is completed on every product that is being placed on the market by the manufacturer which would discover any breaches of the regulation. The government of india through the drug and cosmetic act 1940 and rules 1945 has made stringent provisions regarding import manufacturing sale and distribution of cosmetics. Moreover it strengthens certain elements of the regulatory framework for cosmetics such.
Conditions of license c. These provisions mainly cover the licensing for the. Regulation ec no 12232009 of the european parliament and of the council of 30 november 2009 on cosmetic products recast text with eea relevance.
Https Eur Lex Europa Eu Legal Content En Txt Pdf Uri Celex 32013d0674 From En Cosmetic Brands Vietnam